What Is Evaporative Cooling?
Cooling through evaporation is a natural occurrence. The most common example we all experience is perspiration, or sweat. As perspiration evaporates it absorbs heat to cool your body.
The principle underlying evaporative cooling is the fact that water must have heat applied to it to change from a liquid to a vapor. When evaporation occurs, this heat is taken from the water that remains in the liquid state, resulting in a cooler liquid.
Evaporative cooling systems use the same principle as perspiration to provide cooling for machinery and buildings. A cooling tower is a heat-rejection device, which discharges warm air from the cooling tower to the atmosphere through the cooling of water. In the HVAC industry, the term “cooling tower” is used to describe both open and closed circuit heat rejection equipment.
In an HVAC system, heat is generated by the sun shining on the building, the computers, and people. The heat is picked up in the air handlers which are indirectly tied to the refrigerant through several heat exchangers. The heat boils the refrigerant from a liquid to a vapor. Cooling Tower water is circulated through a heat exchanger where refrigerant vapor is condensed and heat is transferred to the water. The purpose of the cooling towers is to cool the warm water returning from the heat exchanger so it can be reused. In the open cooling tower, the warm return water from the heat exchanger is sprayed over the “fill”. The fill provides the surface area to enhance the heat transfer between the water and air, causing a portion of the water to evaporate. That cool water then loops back to the beginning of the process, to absorb more heat from the heat exchanger.
In a closed circuit cooling tower, cold water or a solution of ethylene or propylene glycol is used to provide cooling. Unlike in an open cooling tower, the fluid used to provide cooling is enclosed in a coil and is not exposed directly to the air. Cold water is recirculated over the outside of the coil, which contains the fluid that has been heated by the process. During operation, heat is transferred from the fluid through the coil to the spray water and then to the atmosphere as a portion of the water evaporates. The cool fluid in the coil then loops back to the beginning of the process, to be reused in the process.
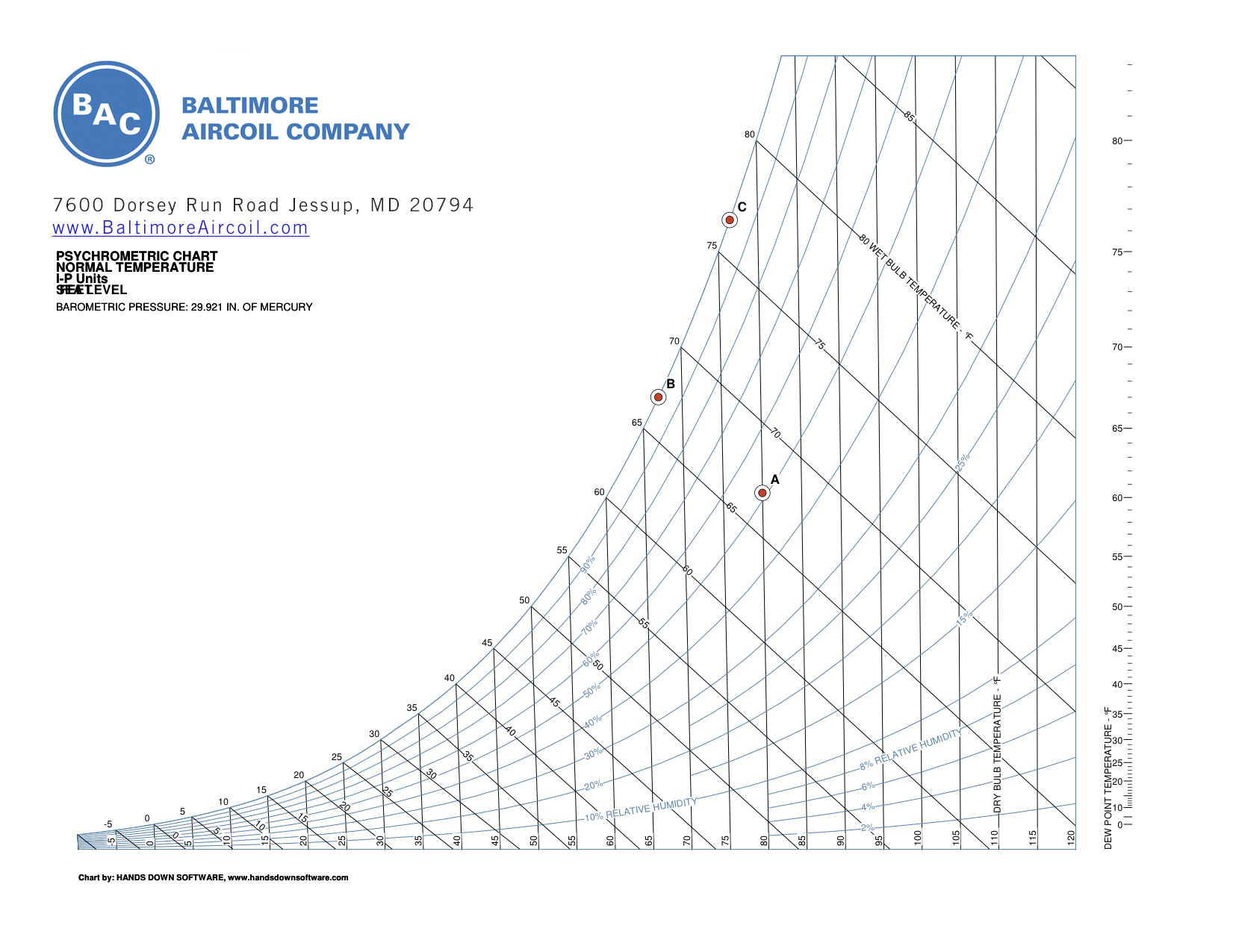
A ton of air-conditioning is the rejection of 12,000 BTUH. A cooling tower ton actually rejects about 15,000 BTUH due to the heat-equivalent of the energy needed to drive the chiller’s compressor. A cooling tower ton is defined as the heat rejection in cooling 3 GPM of water entering at 95°F and leaving the cooling tower at 85°F, with an entering wet bulb temperature of 78°F, which amounts to 15,000 BTUH. The figure below shows the relationship between water and air as they pass through a cooling tower. The curve indicates the drop in water temperature (point A to B) and the rise in the air wet bulb temperature (Point C to D) in their respective passages through the cooling tower.
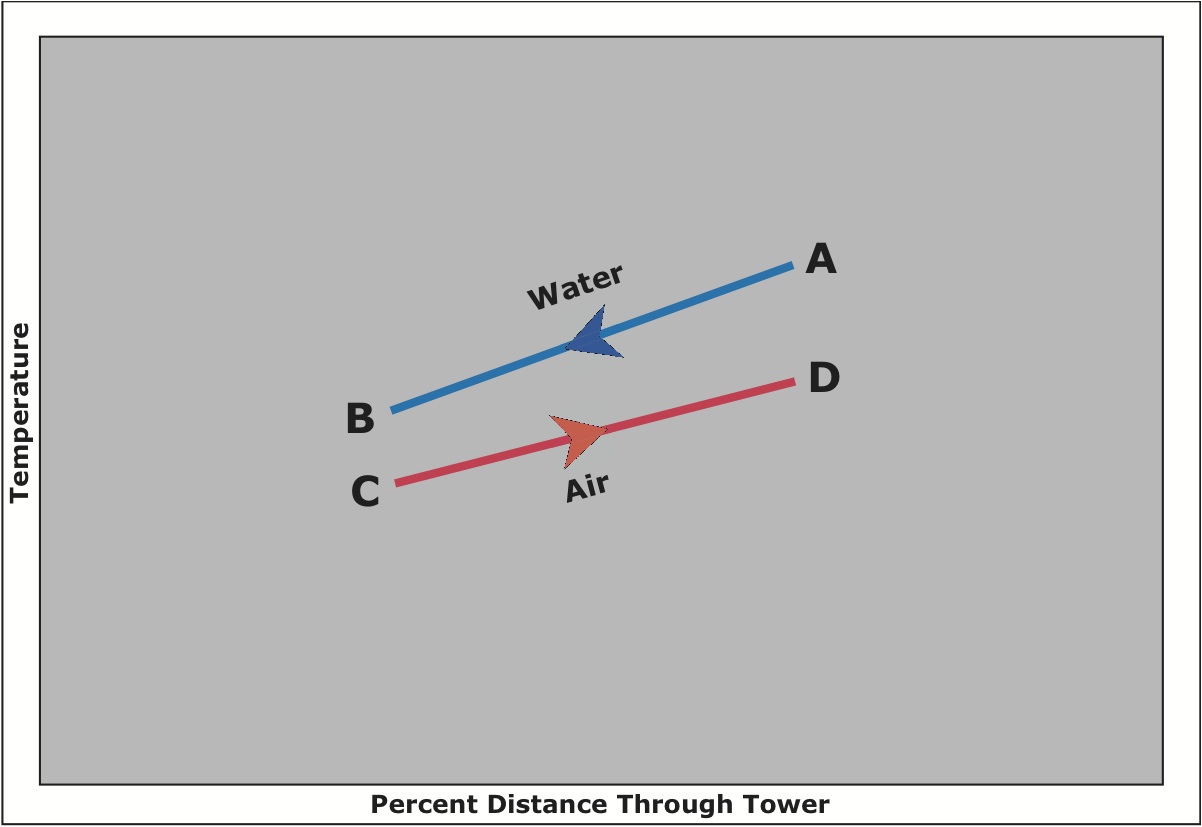
From a heat transfer standpoint, a cooling tower’s performance while cooling a given quantity of water is influenced only by the wet bulb temperature of the entering air. This is clearly indicated in the psychrometric analysis of the air path in a cooling tower as indicated below. The true path is approximated by the dotted curved line from Point A to Point C. To simplify the air path for purposes of explanation, it is broken down into Line AB and BC. In the analysis, air enters the tower at an unsaturated condition (Point A). Before reaching the fill, it is saturated adiabatically as it travels to point B. Passing through the fill, it absorbs heat from the falling water, thereby increasing the total heat content of the air. Since the air is continually being washed with falling water, the process follows the saturation line to the final temperature of the air leaving the tower, Point C.